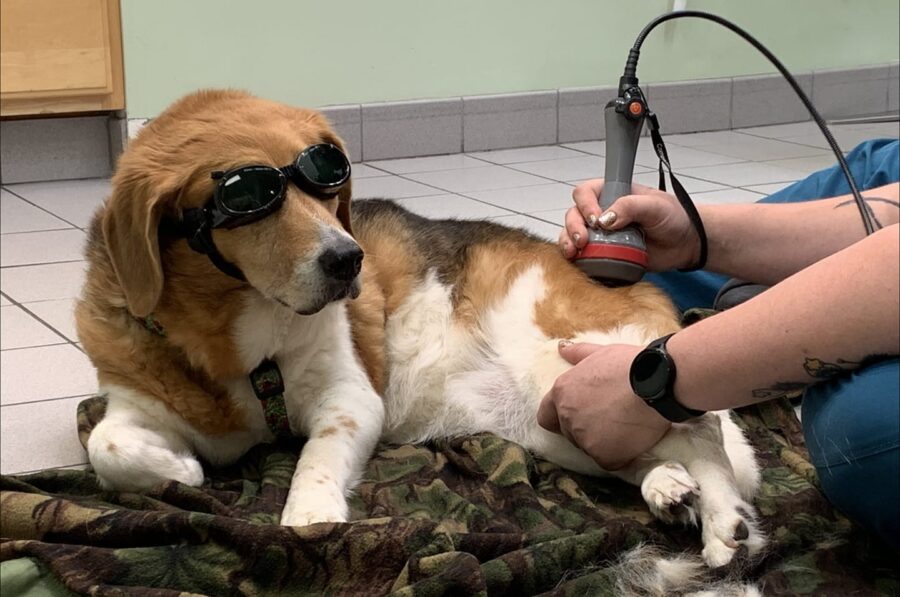
Why laser therapy, or photobiomodulation (PBM) needs to be an essential part of your veterinary treatment plans.
Light as a restorative and protective therapy for the treatment of injury and disease in veterinary medicine has generated increasing interest over the last decade. This is due to numerous factors, especially the ability to apply this modality in a non-invasive way that has no detrimental side effects to the patient, or negative environmental impacts. Laser therapy, or photobiomodulation (PBM), has a beneficial effect on cells and tissues, contributing to a directed modulation of cell behaviors, enhancing the processes of tissue repair and cell proliferation while also reducing inflammation and pain. All these effects make PBM a versatile modality for many “first-aid” situations, such as treating traumatic wounds or burns, acute muscle injuries, and snake bite envenomation cases.
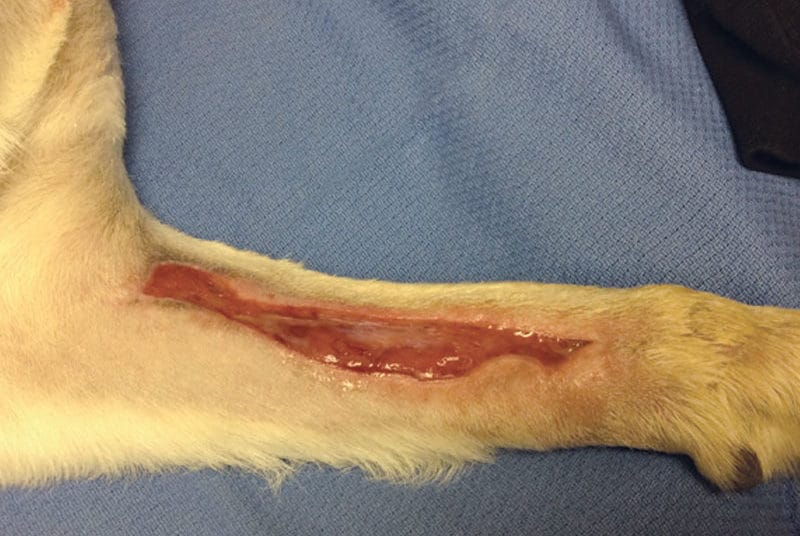
Traumatic wounds, burns and decubital ulcers
The benefits of laser therapy in wound healing are well described in the literature. In vivo and in vitro studies have shown that PBM accelerates the activity of fibroblasts, collagen synthesis, and neovascularization, decreases inflammatory cells, and increases the amount of elastic fibers in the wound healing process.1,2,3 PBM also offers a demonstrated benefit for use in thermal burns to encourage re-epithelialization and reduce scar tissue.4 It may also have potential benefits for infected wounds, where it may enhance macrophage function and modulate the immune response5 as well as potentially having some direct effects on microorganisms.6
Straightforward acute wounds that have been cleaned may require only one to three treatment sessions, depending on wound size, before significant contraction and/or complete healing is noted. Treatment should be administered in an off-contact manner so as not to contaminate the wound further, or contaminate the optical probe itself. The entire wound “bed” should be treated as well as a few inches of healthy-looking tissue margin around the wound.
 A starting dose of 2-5 J/cm2 is recommended, treating daily if possible and then reducing the frequency of treatment sessions as the lesion improves.7 Chronic or infected wounds and thermal burns may require a more aggressive treatment schedule. Alongside other standard of care therapies and supportive care measures, especially for wounds such as decubital ulcers, higher fluences (energy densities) may be necessary in order to appreciate beneficial results. If no improvements are seen after three or four treatment sessions with the previously mentioned starting doses, an increase in the amount of total energy (in total joules) delivered at 25% to 50% per session is recommended until a significant response is seen.8 It is worth noting that PBM should be applied after the wound or burn has been cleaned (or debrided) and prior to the application of any dressings, ointments, or bandages.
A starting dose of 2-5 J/cm2 is recommended, treating daily if possible and then reducing the frequency of treatment sessions as the lesion improves.7 Chronic or infected wounds and thermal burns may require a more aggressive treatment schedule. Alongside other standard of care therapies and supportive care measures, especially for wounds such as decubital ulcers, higher fluences (energy densities) may be necessary in order to appreciate beneficial results. If no improvements are seen after three or four treatment sessions with the previously mentioned starting doses, an increase in the amount of total energy (in total joules) delivered at 25% to 50% per session is recommended until a significant response is seen.8 It is worth noting that PBM should be applied after the wound or burn has been cleaned (or debrided) and prior to the application of any dressings, ointments, or bandages.
Muscle injuries
PBM may be used preventatively to benefit athletic training by reducing delayed-onset muscle soreness and signs of muscle damage after intense exercise. This modality may also be used to treat muscle damage caused by strains or trauma. Numerous studies have demonstrated the usefulness of PBM in muscle recovery after injury.8,9,10 Studies that have examined markers for oxidative stress and inflammation in muscle tissue from euthanized animals, or in serum from other patients, have demonstrated that PBM accelerated or resolved the acute inflammatory response and reduced oxidative stress elicited by muscle trauma.11,12 Other research that looked at PBM effects on histopathological features after muscle injury showed that PBM attenuated the extent of edema, myofibrillar degeneration, and area of necrosis.13
Taking all of the above into account, PBM is crucial during several phases of rehabilitation from muscle injury. During the acute phase following injury, where the goal is to limit the effects of immobilization, reduce pain and inflammation, and promote healing of the injured tissue, PBM may be used daily if possible.14 Additionally, it may be used throughout later stages of rehabilitation where the goals shift to enhancing mobility, improving endurance and/or strength, re-establishing more normal neuromuscular control patterns, and ultimately to strengthening and conditioning injured areas even further. Time between treatment sessions is gradually increased as the patient improves, a common approach to resolving injuries and returning to full function. In the case of performance animals, long term maintenance therapy as a preventative measure prior to athletic events may also be considered.
When treating in vivo, the depth of the target tissue is of utmost importance.15 Thus, for transcutaneously delivered light to be effective in injured muscle tissue, the light’s parameters and PBM application protocols must be such that — after accounting for the light’s energy losses in the haircoat and the intervening tissues of the skin, fat, etc. — a sufficient amount of light must reach and be absorbed by muscle cells in the injured area(s). A starting point for fluences that may be effective, depending on the size of the patient and the depth of tissue being treated, would be 6-10 J/cm2 up to 20 J/cm2 or higher in some cases.15,18,19 One way to maximize the amount of light penetrating to deep tissue for musculoskeletal injuries is to treat with the optical probe in firm contact with the skin, minimizing the amount of light that may be lost due to reflection off the skin’s surface.17
 Snake bites
Snake bites
Numerous small animal models have been researched with regards to PBM and inflammatory pain. One particular application of PBM and its benefits in treating inflammatory pain is related directly to snake bite envenomation. Bites from certain snakes can cause severe local tissue damage, systemic coagulopathy, and localized edema, intense pain, hemorrhage, and myonecrosis. Studies in mice have shown that PBM improved pain, reduced inflammatory infiltration, stimulated phagocytosis, and increased regeneration of muscle fibers and myoblast proliferation after injection of Bothrops moojeni venom.20,21 PBM has also been shown to promote vascular endothelial growth factor receptor 1 (VEGFR-1) expression and increase angiogenesis,22 as well as improve skeletal muscle regeneration by accelerating recovery of the myofiber mass23 post-injection of venom into mouse skeletal muscle.
A veterinary surgeon working from a large multi-specialty 24-hour emergency and critical care hospital in Tucson, Arizona, that treats an average of 395 rattlesnake envenomation cases each year, recently authored a chapter in the veterinary textbook Laser Therapy in Veterinary Medicine: Photobiomodulation.24 In this chapter, Dr. Barbara Gores describes initiating laser therapy for snake bite envenomation patients after they have been stabilized with IV fluid therapy, analgesics, and in most cases, antivenin. Treatment with PBM usually begins six to eight hours after hospital admission and is administered once daily while the patient is hospitalized (usually two to four treatments in-clinic) followed by an additional two to four sessions after discharge at a fluence of approximately 8 J/cm2; though as with other deep tissue doses, this varies depending on the size of the patient and the area/depth of the wound being treated. Dr. Gores goes on to describe that anecdotally, over the past decade since initiating PBM for these cases, there has been a significant decrease in clinical morbidity as well as the necessity to perform major surgical reconstruction of snake bite wounds at their facility. This is also consistent with the anecdotal success reported to this author by other colleagues utilizing PBM in similar cases throughout the country.
In conclusion, since Mester’s original discovery in the 1960s (see sidebar above), a large amount of research has been done to elucidate the mechanisms behind PBM. Though further work focusing on a deeper understanding of molecular mechanisms, biological context, and optimal dosing parameters for various conditions is still underway, great progress has been made in PBM research. This modality has the potential to make a significant impact on the overall prognosis and outcome of many veterinary patients being treated for acute traumatic conditions; however, this therapy may be commonly overlooked when evaluating these cases. Laser therapy is well tolerated in veterinary patients, and easy to perform by technical staff. Its use should be considered, especially in these types of cases, in order to maximize success, reduce pain, and hasten the return to full health.
References
1Mester, E, et al. The effect of laser beams on the growth of hair in mice. Radiobiol. Radiother, 1968. 9(5), 621–626.
2Freitas and Hamblin. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J Sel Top Quantum Electron, 2016. 22(3).
3Melo VA, et al. Effect of low level laser on sutured wound healing in rats. Acta Cir Bras. 2011. 26(2): 129-134.
4Loreti, EH, et al. Use of Laser Therapy in the Healing Process: A Literature Review. Photomed Laser Surg, 2015. 33(2): 104-116.
5Gal, P, et al. Histological assessment of the effect of laser irradiation on skin wound healing in rats. Photomed Laser Surg, 2006. 24(4): 480-488.
6De Moraes, JM, et al. Anti-inflammatory effect of low intensity laser on the healing of third degree burn wounds in rats, 2013. 28(4): 1169-76.
7Burger, E, et al. Low level laser therapy to the mouse femur enhances the fungicidal response of neutrophils against Paracoccidioides brasiliensis. PLoS Negl Trop Dis, 2015. 9(2).
8Bradley, D. Wounds In: Laser Therapy in Veterinary Medicine: Photobiomodulation. Eds. Ronald J Riegel and John C Godbold. 2017. John Wiley & Sons, West Sussex, UK. 100-113.
9Nussbaum, EL, et al. Effects of low level laser of 810nm upon in vitro growth of bacteria: relevance of irradiance and radiant exposure. J Clin Laser Med Surg, 2003. 21(5)283-290.
10Ferraresi, C, et al. Effects of Light-emitting diode therapy on muscle hypertrophy, gene expression, performance, damage, and delayed-onset muscle soreness: Case control study with a pair of identical twins. Am J Phys Med Rehabil, 2016. 95(10): 746-757.
11Sussai DA, et al. Low-level laser therapy attenuates creatine kinase levels and apoptosis during forced swimming in rats. Lasers Med Sci, 2010. 25:115–120.
12Borsa PA, Larkin KA, True JM. Does phototherapy enhance skeletal muscle contractile function and postexercise recovery? A systematic review. J Athl Train, 2013. 48: 57–67.
13De Marchi T, et al. Low-level laser therapy (LLLT) in human progressive-intensity running: effects on exercise performance, skeletal muscle status, and oxidative stress. Lasers Med Sci, 2012. 27:231–236.
14Silveira PCL, et al. Low-level laser therapy attenuates the acute inflammatory response induced by muscle traumatic injury. Free Radic Res, 2016. 50:503–513.
15Renno et al. The effects of Low Level Laser Therapy on Injured Skeletal Muscle. Bras Archives Biol Tech, 2014. 57(1): 48-54.
16Canapp, D. Laser Therapy for the Canine Athlete In: Laser Therapy in Veterinary Medicine: Photobiomodulation. Eds. Ronald J Riegel and John C Godbold. 2017. John Wiley & Sons, West Sussex, UK. 215-226.
17Zein R, et al. Review of light parameters and photobiomodulation efficacy: dive into complexity. J Biomed Optics, 2018. 23(12).
18Gaynor J. Energy modalities, therapeutic laser and pulsed electromagnetic field therapy. In: Handbook of Veterinary Pain Management, 3rd Ed. 2015. Mosby, St. Louis, MO. 357-362.
19Miller L, et al. Retrospective Observational Study and Analysis of Two Different Photobiomodulation Therapy Protocols Combined with Rehabilitation Therapy as Therapeutic Interventions for Canine Degenerative Myelopathy. Photobiomodulation, Photomedicine, and Laser Surg, 2020. 38(4): 195-205.
20Nadur-Andrande, N, et al. Effects of photobiomodulation on edema and hemorrhage induced by Bothrops moojeni venom. Lasers Med Sci, 2012. 27(1): 65-70.
21Dourado, DM,et al. Effects of Ga-As laser irradiation on myonecrosis caused by Bothrops Moojeni snake venom. Lasers Surg Med, 2003. 33(5): 352-357.
22Dourado, DM, et al. Low level laser therapy promotes vascular endothelial growth factor receptor 1 expression expression in endothelial and non-endothelial cells of mice gastrocnemius exposed to snake venom. Photochem Photobiol, 2011. 87(2): 418-426.
23Silva, LM, et al. Photobiomodulation protects and promotes differentiation of C2C12 myoblast cells exposed to snake venom. PLoS One, 2012. 11(4).
24Gores, B. Snake Bites In: Laser Therapy in Veterinary Medicine: Photobiomodulation. Eds. Ronald J Riegel and John C Godbold. 2017. John Wiley & Sons, West Sussex, UK. 128-131.